Multiple Choice
The energy of a one-electron atom or ion is given by 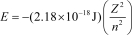 ,where Z is the atomic number of the element.Which of the following one-electron ions would require the most energy to remove its electron?
,where Z is the atomic number of the element.Which of the following one-electron ions would require the most energy to remove its electron?
A) Li2+
B) Be3+
C) He+
D) B4+
E) C5+
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q5: Which of the following is the correct
Q6: The de Broglie wavelength of buckyballs
Q8: Consider the radial distribution shown below for
Q9: Based on the following data and
Q12: Copper commonly forms a cation with a
Q13: Which of the following sets of quantum
Q14: If light at 193 nm from
Q15: The energy change for an electronic transition
Q29: Atomic spectra are due to the changes
Q113: Which of the following elements would you