Essay
Consider the radial distribution shown below for an electron in an s atomic orbital where r is the distance of the electron from the nucleus. 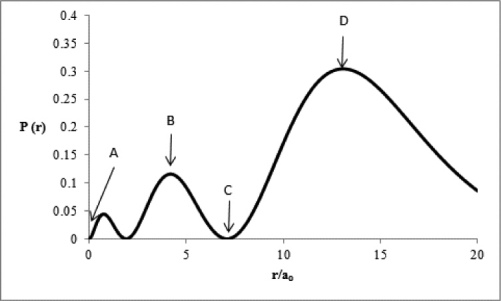 Identify: (1)where the atomic nucleus is located; (2)a radial node; (3)the most probable distance of the electron from the nucleus; and (4)the principal quantum number for this orbital.
Identify: (1)where the atomic nucleus is located; (2)a radial node; (3)the most probable distance of the electron from the nucleus; and (4)the principal quantum number for this orbital.
Correct Answer:

Verified
(1)The nucleus is located at A...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q3: The theoretical work of Max Planck on
Q5: Which of the following is the correct
Q6: The de Broglie wavelength of buckyballs
Q9: Based on the following data and
Q10: The energy of a one-electron atom or
Q12: Copper commonly forms a cation with a
Q13: Which of the following sets of quantum
Q29: Atomic spectra are due to the changes
Q113: Which of the following elements would you
Q154: Why is the electron not likely to