Multiple Choice
Which of the following sets of quantum numbers is allowed?
A) n = 2,  = 2,m
= 2,m  = 0,ms = -
= 0,ms = - 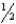
B) n = 7,  = 0,m
= 0,m  = 0,ms = +
= 0,ms = + 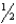
C) n = 7,  = -1,m
= -1,m  = -1,ms = -
= -1,ms = - 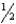
D) n = 1,  = 2,m
= 2,m  = 0,ms = +
= 0,ms = + 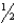
E) n = 5,  = 6,m
= 6,m  = -2,ms = -
= -2,ms = - 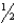
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q8: Consider the radial distribution shown below for
Q9: Based on the following data and
Q10: The energy of a one-electron atom or
Q12: Copper commonly forms a cation with a
Q14: If light at 193 nm from
Q15: The energy change for an electronic transition
Q18: What is the approximate uncertainty in
Q80: Which of the following will lead to
Q113: Which of the following elements would you
Q140: Which statement about electromagnetic radiation is NOT