Multiple Choice
Which set of quantum numbers could be correct for the highest energy electron in barium,Ba?
A) n = 6,  = 1,m
= 1,m  = -1,ms = -
= -1,ms = - 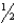
B) n = 6,  = 0,m
= 0,m  = -1,ms = +
= -1,ms = + 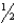
C) n = 6,  = 1,m
= 1,m  = 0,ms = -
= 0,ms = - 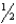
D) n = 6,  = 2,m
= 2,m  = 0,ms = +
= 0,ms = + 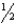
E) n = 6,  = 0,m
= 0,m  = 0,ms = -
= 0,ms = - 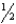
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q17: What wavelength of light is required to
Q80: Electromagnetic radiation with a frequency of 8.6
Q81: De Broglie reasoned that for the electron
Q97: What is meant when two or more
Q107: Which of the following statements about the
Q108: Using the periodic table,predict which element is
Q111: You think you have identified a
Q113: A shell is defined by _<br>A)the <img
Q114: An electron _ will have the
Q132: Which of the following metals would be