Multiple Choice
The molar solubility of PbI2 is 1.52 10-3 M.Calculate the value of Ksp for PbI2.
A) 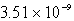
B) 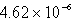
C) 
D) 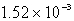
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: Given the following K<sub>sp</sub> values,which statement about
Q3: In the qualitative analysis scheme for metal
Q4: The best explanation for the dissolution of
Q5: The K<sub>sp</sub> for Mn(OH)<sub>2</sub> is 2.0
Q6: The following questions refer to the
Q7: Determine the equilibrium concentration of the
Q8: The solubility of La(IO<sub>3</sub>)<sub>3</sub> in a 0.42
Q9: The solubility of silver phosphate,Ag<sub>3</sub>PO<sub>4,</sub> at
Q10: Calculate the solubility of Ca<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub> (K<sub>sp</sub>
Q11: An unknown salt,M<sub>2</sub>Z,has a K<sub>sp</sub> of <img