Multiple Choice
The solubility of CaSO4 in pure water at 0oC is 1.14 gram(s) per liter.The value of the solubility product is
A) 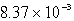
B) 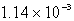
C) 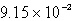
D) 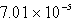
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q10: Calculate the solubility of Ca<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub> (K<sub>sp</sub>
Q11: An unknown salt,M<sub>2</sub>Z,has a K<sub>sp</sub> of <img
Q12: Consider a solution containing the following cations:
Q13: Calculate the solubility of Ag<sub>2</sub>CrO<sub>4</sub> (K<sub>sp</sub>
Q14: An industrial plant processes its waste
Q16: Given the following values of equilibrium
Q17: A solution contains 0.018 moles each of
Q18: What is the maximum concentration of
Q19: The overall K<sub>f</sub> for the complex
Q20: In the qualitative analysis scheme for metal