Multiple Choice
Given the following values of equilibrium constants: Cu(OH) 2(s)  Cu2+(aq) + 2OH-(aq)
Cu2+(aq) + 2OH-(aq)
Ksp = 1.60 10-19
Cu(NH3) 42+(aq)  Cu2+(aq) + 4NH3(aq)
Cu2+(aq) + 4NH3(aq)
K = 1.0 10-13
What is the value of the equilibrium constant for the following reaction?
Cu(OH) 2(s) + 4NH3(aq)  Cu(NH3) 42+(aq) + 2OH-(aq)
Cu(NH3) 42+(aq) + 2OH-(aq)
A) 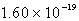
B) 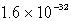
C) 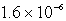
D) 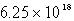
E) 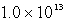
Correct Answer:

Verified
Correct Answer:
Verified
Q11: An unknown salt,M<sub>2</sub>Z,has a K<sub>sp</sub> of <img
Q12: Consider a solution containing the following cations:
Q13: Calculate the solubility of Ag<sub>2</sub>CrO<sub>4</sub> (K<sub>sp</sub>
Q14: An industrial plant processes its waste
Q15: The solubility of CaSO<sub>4</sub> in pure water
Q17: A solution contains 0.018 moles each of
Q18: What is the maximum concentration of
Q19: The overall K<sub>f</sub> for the complex
Q20: In the qualitative analysis scheme for metal
Q21: Barium carbonate has a measured solubility