Multiple Choice
A solution contains 0.018 moles each of I-,Br-,and Cl-.When the solution is mixed with 200 mL of 0.24 M AgNO3,how much AgCl(s) precipitates out? 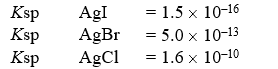
A) 0.0 g
B) 1.7 g
C) 2.6 g
D) 3.3 g
E) 5.0 g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q12: Consider a solution containing the following cations:
Q13: Calculate the solubility of Ag<sub>2</sub>CrO<sub>4</sub> (K<sub>sp</sub>
Q14: An industrial plant processes its waste
Q15: The solubility of CaSO<sub>4</sub> in pure water
Q16: Given the following values of equilibrium
Q18: What is the maximum concentration of
Q19: The overall K<sub>f</sub> for the complex
Q20: In the qualitative analysis scheme for metal
Q21: Barium carbonate has a measured solubility
Q22: Which of the following compounds has