Multiple Choice
The solubility of Cd(OH) 2 in water is 1.67 10-5 mol/L.The Ksp value for Cd(OH) 2 is
A) 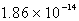
B) 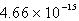
C) 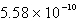
D) 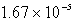
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q49: The K<sub>sp</sub> of PbSO<sub>4</sub> is 1.3
Q50: Sodium chloride is added slowly to
Q51: The molar solubility of BaCO<sub>3</sub> (K<sub>sp</sub>
Q52: The solubility of an unknown salt,M<sub>3</sub>Z<sub>2,</sub> at
Q53: The solubility of AgCl in water is
Q55: A 100.-mL sample of solution contains
Q56: The concentration of Mg<sup>2+</sup> in seawater
Q57: A 50.0-mL sample of 0.100 M
Q58: The solubility of an unknown salt,MZ<sub>2,</sub> at
Q59: The K<sub>sp</sub> of an unknown salt,MZ<sub>2</sub>,is <img