Multiple Choice
In the reaction below, ________ is the oxidizing agent. 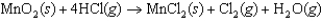
A) MnO2(s)
B) HCl(g)
C) MnCl2(s)
D) Cl2(g)
E) H2O(g)
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q41: Because of recent advances in recovery technology,
Q42: For the following reaction, predict the pH
Q43: Which one of the following items does
Q44: The diagram below represents a voltaic cell.
Q45: For the following reaction, predict the pH
Q47: A voltaic cell is constructed based on
Q48: An electrochemical cell at 298 K is
Q49: The capacity of a battery usually is
Q50: When aluminum metal is obtained from aluminum
Q51: Oxidation is the _<br>A)gain of electrons.<br>B)loss of