Multiple Choice
A voltaic cell is constructed based on the oxidation of zinc metal and the reduction of silver cations. Solutions of silver nitrate and zinc nitrate also were used. Locate the silver nitrate on the diagram. 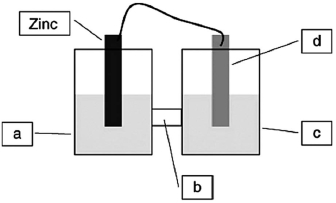
A) a
B) b
C) c
D) d
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q42: For the following reaction, predict the pH
Q43: Which one of the following items does
Q44: The diagram below represents a voltaic cell.
Q45: For the following reaction, predict the pH
Q46: In the reaction below, _ is the
Q48: An electrochemical cell at 298 K is
Q49: The capacity of a battery usually is
Q50: When aluminum metal is obtained from aluminum
Q51: Oxidation is the _<br>A)gain of electrons.<br>B)loss of
Q52: The following reaction is called the "super