Multiple Choice
Use the table of standard reduction potentials below to identify the metal or metal ion that is the strongest reducing agent. Standard Reduction
Potentials (volts) in Aqueous Solution 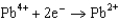 1.80
1.80 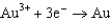 1.50
1.50 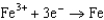 0.771
0.771 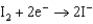 0.535
0.535 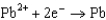 0.124
0.124 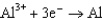 1.66
1.66 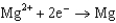 2.37
2.37 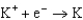 2.93
2.93
A) Pb4
B) Pb2
C) K
D) K
E) Al
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q121: Which of the following is not an
Q122: What is the cell potential for this
Q123: A NiMH battery uses _ as the
Q124: A typical AA battery has a capacity
Q125: If the free-energy change of the following
Q127: The capacity of a battery usually is
Q128: Oxidation refers to _<br>A)an increase in oxidation
Q129: The oxidation of hydrogen by oxygen is
Q130: How long would it take to electroplate
Q131: The numerical value of the Faraday constant