Multiple Choice
Consider the following standard reduction potentials. Reduction Half-Reaction 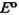 (volts)
(volts) 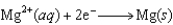 2.38
2.38 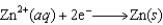 0.76
0.76 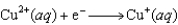 0.16
0.16
The Mg/Mg2 half-reaction can be paired with the other two to produce voltaic cells because ________
A) Mg is a powerful oxidizing agent.
B) Mg is a powerful reducing agent.
C) Mg2 is a powerful reducing agent.
D) Mg2 is a powerful oxidizing agent.
E) Zn and Cu are readily oxidized.
Correct Answer:

Verified
Correct Answer:
Verified
Q83: Which statement regarding battery-powered electric cars is
Q84: Copper metal is purified by electrolysis. How
Q85: The energy supplied by a battery can
Q86: A voltaic cell is constructed based on
Q87: Use the table of standard reduction potentials
Q89: The diagram below represents a voltaic cell.
Q90: Sodium carbonate is produced using the Solvay
Q91: Which one of the following is not
Q92: What must be true about the standard
Q93: In the following reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="In