Essay
What must be true about the standard reduction potentials for the following reaction to proceed spontaneously? 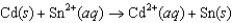
Correct Answer:

Verified
The standard reduction potenti...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q87: Use the table of standard reduction potentials
Q88: Consider the following standard reduction potentials. Reduction
Q89: The diagram below represents a voltaic cell.
Q90: Sodium carbonate is produced using the Solvay
Q91: Which one of the following is not
Q93: In the following reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="In
Q94: If in using a lead-acid battery to
Q95: Reduction is the _<br>A)gain of electrons.<br>B)loss of
Q96: A voltaic cell is constructed based on
Q97: How does a fuel cell differ from