Multiple Choice
Which statement does not correctly describe a standard hydrogen electrode (SHE) ?
A) The SHE is assigned a standard reduction potential of exactly 1 V.
B) 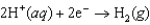
C) Pt|H2(g, 1atm) |H(aq, 1 M ) ||
D) ||H(aq, 1 M ) |H2(g, 1atm) |Pt
E) The SHE consists of a platinum electrode immersed in an acid solution and a stream of hydrogen gas.
Correct Answer:

Verified
Correct Answer:
Verified
Q93: In the following reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="In
Q94: If in using a lead-acid battery to
Q95: Reduction is the _<br>A)gain of electrons.<br>B)loss of
Q96: A voltaic cell is constructed based on
Q97: How does a fuel cell differ from
Q99: Neuron cells generate electrical signals by concentration
Q100: The standard cell potential for the nickel-cadmium
Q101: You have a job as a summer
Q102: A voltaic cell is constructed based on
Q103: For the following electrochemical cell, draw and