Multiple Choice
A voltaic cell is constructed based on the oxidation of zinc metal and the reduction of silver cations. Solutions of silver nitrate and zinc nitrate also were used. Locate the salt bridge in the diagram. 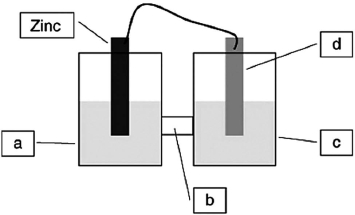
A) a
B) b
C) c
D) d
Correct Answer:

Verified
Correct Answer:
Verified
Q91: Which one of the following is not
Q92: What must be true about the standard
Q93: In the following reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="In
Q94: If in using a lead-acid battery to
Q95: Reduction is the _<br>A)gain of electrons.<br>B)loss of
Q97: How does a fuel cell differ from
Q98: Which statement does not correctly describe a
Q99: Neuron cells generate electrical signals by concentration
Q100: The standard cell potential for the nickel-cadmium
Q101: You have a job as a summer