Multiple Choice
The standard cell potential for the nickel-cadmium battery is 1.35 V, and the cell reaction can be written as 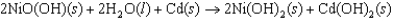 Which one of the following statements do you expect to be true based on the Nernst equation? Note Q reaction quotient, and K equilibrium constant for the cell reaction.
Which one of the following statements do you expect to be true based on the Nernst equation? Note Q reaction quotient, and K equilibrium constant for the cell reaction.
A) As the battery is used, the cell voltage approaches zero because Q approaches K in value.
B) When the battery no longer works, the cell voltage is zero because Q K.
C) As the battery is used, the cell voltage does not change because Q equals 1.
D) When the battery is fully charged, Q K.
E) When the battery is fully charged, Q K.
Correct Answer:

Verified
Correct Answer:
Verified
Q95: Reduction is the _<br>A)gain of electrons.<br>B)loss of
Q96: A voltaic cell is constructed based on
Q97: How does a fuel cell differ from
Q98: Which statement does not correctly describe a
Q99: Neuron cells generate electrical signals by concentration
Q101: You have a job as a summer
Q102: A voltaic cell is constructed based on
Q103: For the following electrochemical cell, draw and
Q104: Based on the information in the table
Q105: The unit of current, ampere (A), is