Essay
The following reaction is called the "super iron reaction." In this reaction, identify
(1) the species oxidized,
(2) the species reduced, and
(3) the number of electrons transferred for the reaction as written, and
(4) explain why it is called the "super iron reaction." 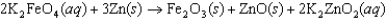
Correct Answer:

Verified
(1) Zinc metal is oxidized to Zn(II).
(2...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
(2...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q47: A voltaic cell is constructed based on
Q48: An electrochemical cell at 298 K is
Q49: The capacity of a battery usually is
Q50: When aluminum metal is obtained from aluminum
Q51: Oxidation is the _<br>A)gain of electrons.<br>B)loss of
Q53: Where in the periodic table do you
Q54: What is the standard cell potential for
Q55: What is the most important use for
Q56: What constitutes a standard hydrogen electrode?
Q57: Which statement about a cathode in a