Multiple Choice
Indicate which one of the following reactions results in a negative Ssys.
A) 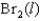
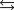
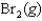
B) 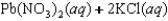
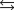
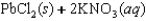
C) 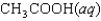
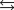
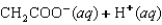
D) 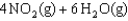
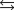
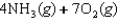
E) 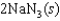
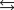
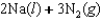
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q98: At what temperature does the Fe(s) <img
Q99: An ice cube at 0<font face="symbol"></font>C melts
Q100: Determine the value of <font face="symbol"></font>G<font face="symbol"></font>
Q101: Hydrogen reacts with nitrogen to form ammonia
Q102: Which statement characterizes the following table? Temperature
Q104: Calcium sulfate is a desiccant used for
Q105: Which of the following processes will lead
Q106: A reaction is at equilibrium at a
Q107: Indicate which one of the following reactions
Q108: The entropy change of the surroundings, <font