Multiple Choice
A reaction is at equilibrium at a given temperature and constant pressure when ________
A) (Srxn 0.)
B) ( 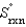 0.)
0.)
C) (Grxn 0.)
D) (  0.)
0.)
E) (Hrxn 0.)
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q101: Hydrogen reacts with nitrogen to form ammonia
Q102: Which statement characterizes the following table? Temperature
Q103: Indicate which one of the following reactions
Q104: Calcium sulfate is a desiccant used for
Q105: Which of the following processes will lead
Q107: Indicate which one of the following reactions
Q108: The entropy change of the surroundings, <font
Q109: What is the value of the equilibrium
Q110: Dinitrogen tetroxide (N<sub>2</sub>O<sub>4</sub>) decomposes to nitrogen dioxide
Q111: A sketch of the free energy for