Multiple Choice
Calcium sulfate is a desiccant used for storage of samples and equipment in a dry atmosphere because it absorbs water from air. The relevant thermodynamic reaction equation is given below. At what temperature will this reaction reverse to release the water and regenerate the dry desiccant? 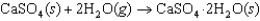 H 104.9 kJ/mol, S 291.2 J/(mol K)
H 104.9 kJ/mol, S 291.2 J/(mol K)
A) 58C
B) 78C
C) 98C
D) 68C
E) 87C
Correct Answer:

Verified
Correct Answer:
Verified
Q99: An ice cube at 0<font face="symbol"></font>C melts
Q100: Determine the value of <font face="symbol"></font>G<font face="symbol"></font>
Q101: Hydrogen reacts with nitrogen to form ammonia
Q102: Which statement characterizes the following table? Temperature
Q103: Indicate which one of the following reactions
Q105: Which of the following processes will lead
Q106: A reaction is at equilibrium at a
Q107: Indicate which one of the following reactions
Q108: The entropy change of the surroundings, <font
Q109: What is the value of the equilibrium