Multiple Choice
Determine 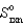 for
for 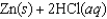
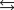
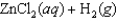 given the following information:
given the following information: 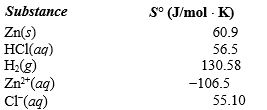
A) (39.6 J/K)
B) 0 J/K
C) (39.6 J/K)
D) (38.2 J/K)
E) (38.2 J/K)
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q107: Indicate which one of the following reactions
Q108: The entropy change of the surroundings, <font
Q109: What is the value of the equilibrium
Q110: Dinitrogen tetroxide (N<sub>2</sub>O<sub>4</sub>) decomposes to nitrogen dioxide
Q111: A sketch of the free energy for
Q113: What is the entropy change if 4.500
Q114: For a particular hypothetical reaction, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg"
Q115: Which of the following must be true
Q116: Which of the following graphs best depicts
Q117: Which of the following will have the