Multiple Choice
A sketch of the free energy for a hypothetical chemical equilibrium is shown here. Which sketch shows the equilibrium position labeled with an asterisk (*) ?
A) 
B) 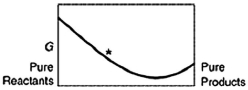
C) 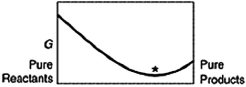
D) 
Correct Answer:

Verified
Correct Answer:
Verified
Q106: A reaction is at equilibrium at a
Q107: Indicate which one of the following reactions
Q108: The entropy change of the surroundings, <font
Q109: What is the value of the equilibrium
Q110: Dinitrogen tetroxide (N<sub>2</sub>O<sub>4</sub>) decomposes to nitrogen dioxide
Q112: Determine <font face="symbol"></font><font face="symbol"></font> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="Determine
Q113: What is the entropy change if 4.500
Q114: For a particular hypothetical reaction, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg"
Q115: Which of the following must be true
Q116: Which of the following graphs best depicts