Multiple Choice
For the following endothermic reaction, predict under which conditions the reaction will be spontaneous. 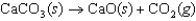
A) The reaction is always spontaneous.
B) The reaction is never spontaneous.
C) The reaction is spontaneous at high temperatures.
D) The reaction is spontaneous at low temperatures.
E) Insufficient data is provided to answer this question.
Correct Answer:

Verified
Correct Answer:
Verified
Q144: Determine the value of <font face="symbol"></font>G<font face="symbol"></font>
Q145: At 0 K, the entropy of a
Q146: The entropy of a NaCl crystal is
Q147: The entropy change in a system (<font
Q148: Determine the normal melting point of benzoic
Q150: Using the thermodynamic data below, determine the
Q151: Determine the value of <font face="symbol"></font>G<font face="symbol"></font>
Q152: A sketch of the free energy for
Q153: The standard molar entropy of silver chloride
Q154: During a spontaneous chemical reaction, it is