Short Answer
Determine the value of G for the reaction at 298 K. 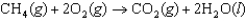 Given
Given 
Correct Answer:

Verified
F...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
F...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Related Questions
Q146: The entropy of a NaCl crystal is
Q147: The entropy change in a system (<font
Q148: Determine the normal melting point of benzoic
Q149: For the following endothermic reaction, predict under
Q150: Using the thermodynamic data below, determine the
Q152: A sketch of the free energy for
Q153: The standard molar entropy of silver chloride
Q154: During a spontaneous chemical reaction, it is
Q155: What is the overall standard free-energy change
Q156: Which statement characterizes the following table? Temperature