Short Answer
Determine the normal melting point of benzoic acid in degrees centigrade given the following data: 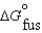 18.02 kJ/mol
18.02 kJ/mol 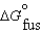 45.56 J/mol K
45.56 J/mol K
Correct Answer:

Verified
122.3F1F1F...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q143: What is the value of the equilibrium
Q144: Determine the value of <font face="symbol"></font>G<font face="symbol"></font>
Q145: At 0 K, the entropy of a
Q146: The entropy of a NaCl crystal is
Q147: The entropy change in a system (<font
Q149: For the following endothermic reaction, predict under
Q150: Using the thermodynamic data below, determine the
Q151: Determine the value of <font face="symbol"></font>G<font face="symbol"></font>
Q152: A sketch of the free energy for
Q153: The standard molar entropy of silver chloride