Multiple Choice
Some pure metals can be obtained from their ores simply by heating to a high temperature to drive off the oxygen, but iron ore usually is refined by reacting it with carbon monoxide. Use the information in the following table to determine whether or not iron ore could be refined by heating to a high temperature and, if so, how high the temperature must be. The oxidation reaction producing iron ore is given below. 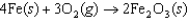 Thermodynamic Properties
Thermodynamic Properties 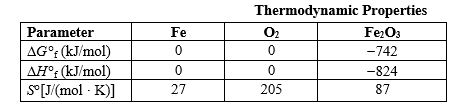
A) Iron ore cannot be refined by heating because the oxidation reaction is spontaneous in the forward direction at all temperatures.
B) Iron ore could be refined by heating, but temperatures in the range 500-1,000 K are needed.
C) Iron ore could be refined by heating, but temperatures in the range 2,000-3,000 K are needed.
D) Iron ore could be refined by heating, but temperatures in the range 1,000-2,000 K are needed.
E) Iron ore could be refined by heating, but the temperature must be greater than 3,000 K.
Correct Answer:

Verified
Correct Answer:
Verified
Q87: The dissolution of ammonium nitrate in water
Q88: If for a given chemical reaction at
Q89: Draw a graph of entropy versus temperature
Q90: Which of the following figures illustrates best
Q91: Nitrogen monoxide molecules can react to form
Q93: Which of the listed perturbations would change
Q94: What is a microstate and how are
Q95: Indicate which of the following has the
Q96: Which of the following processes is/are reversible
Q97: In a biochemical reaction, A <font face="symbol"></font>