Multiple Choice
Which of the listed perturbations would change the value of the equilibrium constant for the following reaction? List those that do as a sequence of letters, for example, ACE.
NH4CO2NH2(s) 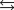 2NH3(g) CO2(g)
2NH3(g) CO2(g)
A) Increasing the quantity of NH4CO2NH2(s)
B) Removing CO2(g)
C) Increasing the total pressure by adding argon gas
D) Increasing the volume of the container
E) Increasing the temperature
Correct Answer:

Verified
Correct Answer:
Verified
Q88: If for a given chemical reaction at
Q89: Draw a graph of entropy versus temperature
Q90: Which of the following figures illustrates best
Q91: Nitrogen monoxide molecules can react to form
Q92: Some pure metals can be obtained from
Q94: What is a microstate and how are
Q95: Indicate which of the following has the
Q96: Which of the following processes is/are reversible
Q97: In a biochemical reaction, A <font face="symbol"></font>
Q98: At what temperature does the Fe(s) <img