Short Answer
Calculate the equilibrium constant at 500 K for the following reaction given the data below:
N2(g) 3H2(g) 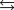 2NH3(g)
2NH3(g)
Kc (298 K) 6.1 105 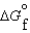 (NH3, g) 16.5 kJ/mol
(NH3, g) 16.5 kJ/mol 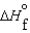 (NH3, g) 46.1 kJ/mol
(NH3, g) 46.1 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Q4: The equilibrium constant for a given reaction
Q5: Determine the entropy change for the reaction
Q6: Which of the following is/are true for
Q7: Consider a closed container containing a 1
Q8: What is the standard entropy change when
Q10: Before class, students were seated at three
Q11: Which statement about the reaction below, where
Q12: Indicate which one of the following reactions
Q13: Which of the following is in the
Q14: The symbol <font face="symbol"></font><font face="symbol"></font> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg"