Multiple Choice
Which statement about the reaction below, where en ethylenediamine (H2NCH2CH2NH2) , is not correct? 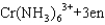
A) The reaction is product-favored because the entropy of the system increases.
B) The Lewis basicity of the amino groups in en is comparable to that of the ammonia molecules.
C) The reaction is reactant-favored because the concentration of ammonia on the right side is higher than the concentration of en on the left side.
D) The reaction enthalpy is small because the Cr-NH3 and Cr-en bond strengths are very similar.
E) Both Cr(NH3) 63 and Cr(en) 33 have an octahedral coordination geometry and a coordination number of 6.
Correct Answer:

Verified
Correct Answer:
Verified
Q6: Which of the following is/are true for
Q7: Consider a closed container containing a 1
Q8: What is the standard entropy change when
Q9: Calculate the equilibrium constant at 500 K
Q10: Before class, students were seated at three
Q12: Indicate which one of the following reactions
Q13: Which of the following is in the
Q14: The symbol <font face="symbol"></font><font face="symbol"></font> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg"
Q15: A sketch of the free energy for
Q16: A reaction is not spontaneous at any