Multiple Choice
Indicate which one of the following reactions results in a positive Ssys.
A) 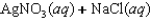
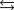
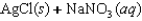
B) 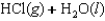
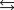
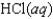
C) 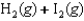
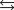
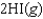
D) 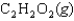
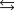
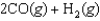
E) 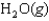
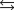
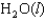
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q7: Consider a closed container containing a 1
Q8: What is the standard entropy change when
Q9: Calculate the equilibrium constant at 500 K
Q10: Before class, students were seated at three
Q11: Which statement about the reaction below, where
Q13: Which of the following is in the
Q14: The symbol <font face="symbol"></font><font face="symbol"></font> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg"
Q15: A sketch of the free energy for
Q16: A reaction is not spontaneous at any
Q17: Care must be taken when dissolving solid