Multiple Choice
Given the van der Waals a constant values for the following gases, which gas has the lowest boiling point?
A) CO2 a  3.64 L2 · atm/mol
3.64 L2 · atm/mol 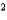
B) Ar a  1.36 L2 · atm/mol
1.36 L2 · atm/mol 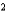
C) CCl4 a  19.75 L2 · atm/mol
19.75 L2 · atm/mol 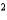
D) HBr a  4.51 L2 · atm/mol
4.51 L2 · atm/mol 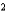
E) CO a  1.51 L2 · atm/mol
1.51 L2 · atm/mol 
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q105: The relative energies (strengths) of the intermolecular
Q106: Which of the following gases would be
Q107: The Henry's law constant (mol/L · atm)
Q108: Point d in the phase diagram below
Q109: A solute is most likely to be
Q111: Of all the noble gases, _ has
Q112: Which of the substances a-d in the
Q113: The density of water decreases as it
Q114: Henry's law constant (mol/L · atm) for
Q115: Of all the noble gases, _ has