Multiple Choice
The pressure of carbon dioxide in a beer bottle is about 2 atm. At this pressure about 0.15 g of carbon dioxide dissolves in 100 mL of beer. How much carbon dioxide remains dissolved in 100 mL after the bottle is opened? The partial pressure of carbon dioxide is 2.0 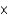 104 atm.
104 atm.
A) 0.15 g
B) 3.8 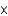 103 g
103 g
C) 6.0 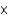 105 g
105 g
D) 3.0 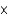 104 g
104 g
E) 1.5 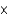 105 g
105 g
Correct Answer:

Verified
Correct Answer:
Verified
Q10: Which one of the following substances would
Q11: The relative energies (strengths) of the intermolecular
Q12: Would water rise to the same height
Q13: Gasoline is primarily a mixture of hydrocarbons
Q14: The temperature at point a in the
Q16: Which liquid, water or ethanol, would you
Q17: Water forms a concave meniscus in a
Q18: Polarizability refers to _<br>A)the ease with which
Q19: The relative energies (strengths) of the intermolecular
Q20: The vapor pressure of a liquid will