Multiple Choice
The relative energies (strengths) of the intermolecular forces present in each of four different pure substances are shown in the figure below. Which substance has the highest melting point? 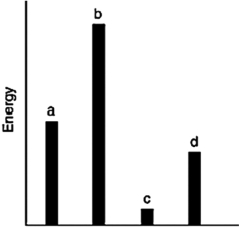
A) a
B) b
C) c
D) d
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q6: Which compound do you predict has the
Q7: Which of the following substances would you
Q8: Which alkane compound has the lowest vapor
Q9: The concept "like dissolves like" explains why<br>A)I<sub>2</sub>
Q10: Which one of the following substances would
Q12: Would water rise to the same height
Q13: Gasoline is primarily a mixture of hydrocarbons
Q14: The temperature at point a in the
Q15: The pressure of carbon dioxide in a
Q16: Which liquid, water or ethanol, would you