Multiple Choice
The temperature at point a in the phase diagram below is the ________ 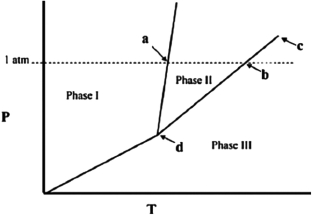
A) critical point.
B) triple point.
C) transition point.
D) normal freezing point.
E) normal boiling point.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: The concept "like dissolves like" explains why<br>A)I<sub>2</sub>
Q10: Which one of the following substances would
Q11: The relative energies (strengths) of the intermolecular
Q12: Would water rise to the same height
Q13: Gasoline is primarily a mixture of hydrocarbons
Q15: The pressure of carbon dioxide in a
Q16: Which liquid, water or ethanol, would you
Q17: Water forms a concave meniscus in a
Q18: Polarizability refers to _<br>A)the ease with which
Q19: The relative energies (strengths) of the intermolecular