Multiple Choice
The phase diagram for carbon dioxide is shown below. What are the phase changes in order as carbon dioxide is heated from 90oC to 50oC, at 500 atm pressure? 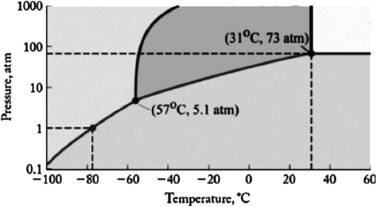
A) solid  liquid
liquid  gas
gas  supercritical fluid
supercritical fluid
B) solid  liquid
liquid  supercritical fluid
supercritical fluid
C) solid  gas
gas
D) gas  liquid
liquid  solid
solid
E) liquid  gas
gas
Correct Answer:

Verified
Correct Answer:
Verified
Q135: The resistance of a liquid to an
Q136: The carbon dioxide pressure in a bottle
Q137: What does the line indicated by the
Q138: Why do the strengths of dispersion interactions
Q139: In understanding why group 16 hydrides, other
Q141: Which of the following pairs of compounds
Q142: Maple syrup is harder to pour out
Q143: The phase diagram for carbon dioxide is
Q144: Which of the following compounds is capable
Q145: Consider the phase diagram for a substance