Multiple Choice
The phase diagram for carbon dioxide is shown below. What are the phase changes in order as carbon dioxide is cooled from 40oC to 70oC, at 200 atm pressure? 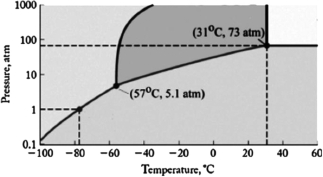
A) supercritical fluid  gas
gas  liquid
liquid  solid
solid
B) gas  solid
solid
C) supercritical fluid  solid
solid
D) supercritical fluid  liquid
liquid  solid
solid
E) gas  liquid
liquid  solid
solid
Correct Answer:

Verified
Correct Answer:
Verified
Q138: Why do the strengths of dispersion interactions
Q139: In understanding why group 16 hydrides, other
Q140: The phase diagram for carbon dioxide is
Q141: Which of the following pairs of compounds
Q142: Maple syrup is harder to pour out
Q144: Which of the following compounds is capable
Q145: Consider the phase diagram for a substance
Q146: The structure of an unsaturated phospholipid is
Q147: The phase diagram for carbon dioxide is
Q148: Which of the following compounds would be