Multiple Choice
Consider the phase diagram for a substance such as the one shown here. If the line between the solid and liquid phases has a negative slope, then the solid phase is ________ than the liquid phase. 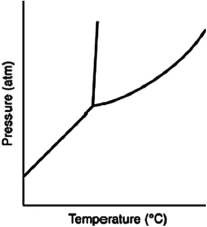
A) more dense
B) less dense
C) more massive
D) less massive
E) hotter
Correct Answer:

Verified
Correct Answer:
Verified
Q140: The phase diagram for carbon dioxide is
Q141: Which of the following pairs of compounds
Q142: Maple syrup is harder to pour out
Q143: The phase diagram for carbon dioxide is
Q144: Which of the following compounds is capable
Q146: The structure of an unsaturated phospholipid is
Q147: The phase diagram for carbon dioxide is
Q148: Which of the following compounds would be
Q149: Why do gases behave nonideally at high
Q150: Under similar conditions, which of the following