Multiple Choice
The phase diagram for carbon dioxide is shown below. What are the phase changes in order as the pressure is increased starting at 0.5 atm, and the temperature is kept at 80oC? 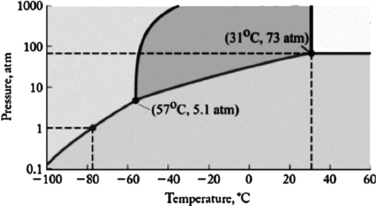
A) solid  gas
gas
B) solid  liquid
liquid  gas
gas
C) liquid  gas
gas
D) gas  solid
solid
E) gas  liquid
liquid
Correct Answer:

Verified
Correct Answer:
Verified
Q142: Maple syrup is harder to pour out
Q143: The phase diagram for carbon dioxide is
Q144: Which of the following compounds is capable
Q145: Consider the phase diagram for a substance
Q146: The structure of an unsaturated phospholipid is
Q148: Which of the following compounds would be
Q149: Why do gases behave nonideally at high
Q150: Under similar conditions, which of the following
Q151: Which is the dominant interaction between oxygen
Q152: Which of the following substances has a