Multiple Choice
The relative energies (strengths) of the intermolecular forces present in each of four different pure gases are shown in the figure below. Which gas will show the smallest deviation from ideal gas behavior? 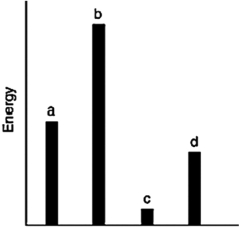
A) a
B) b
C) c
D) d
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q100: Which alkane compound has the lowest boiling
Q101: Define the terms hydrophobic and hydrophilic.
Q102: Which best describes the intermolecular forces present
Q103: The pressure inside a bottle of carbonated
Q104: Which of the following compounds will not
Q106: Which of the following gases would be
Q107: The Henry's law constant (mol/L · atm)
Q108: Point d in the phase diagram below
Q109: A solute is most likely to be
Q110: Given the van der Waals a constant