Multiple Choice
How much energy is required to ionize an He ion in its ground state?
A) 2.18 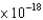 J
J
B) 4.36 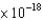 J
J
C) 8.72 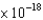 J
J
D) 1.74 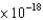 J
J
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q33: An orbital's shape is determined by _<br>A)the
Q34: Which subshell contains six total electrons?<br>A) <img
Q35: The change in energy of a one-electron
Q36: Which transition in a hydrogen atom will
Q37: Electromagnetic radiation with a frequency of 8.6
Q39: Which of the following photons has the
Q40: A coordinate system is defined with orthogonal
Q41: Which of the following objects, all moving
Q42: Indicate which of the following photons can
Q43: Which of the following elements would you