Multiple Choice
The change in energy of a one-electron atom or ion for an electronic transition from the initial energy level ni to the final energy level nf is given by 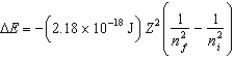 Which of the following species will have the longest wavelength emission line for the transition between the ni 2 and nf 1 levels?
Which of the following species will have the longest wavelength emission line for the transition between the ni 2 and nf 1 levels?
A) H
B) He
C) Li2
D) Be3
E) B4
Correct Answer:

Verified
Correct Answer:
Verified
Q30: Which arrangement is in the correct order
Q31: Which sequence does not correctly arrange the
Q32: Compared with the atomic radius of oxygen
Q33: An orbital's shape is determined by _<br>A)the
Q34: Which subshell contains six total electrons?<br>A) <img
Q36: Which transition in a hydrogen atom will
Q37: Electromagnetic radiation with a frequency of 8.6
Q38: How much energy is required to ionize
Q39: Which of the following photons has the
Q40: A coordinate system is defined with orthogonal