Multiple Choice
An orbital's shape is determined by ________
A) the 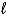 quantum number.
quantum number.
B) the ml quantum number.
C) the ms quantum number.
D) the n quantum number.
E) both the n and 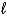 quantum numbers.
quantum numbers.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q28: Which of the following is not a
Q29: Atomic spectra are due to the changes
Q30: Which arrangement is in the correct order
Q31: Which sequence does not correctly arrange the
Q32: Compared with the atomic radius of oxygen
Q34: Which subshell contains six total electrons?<br>A) <img
Q35: The change in energy of a one-electron
Q36: Which transition in a hydrogen atom will
Q37: Electromagnetic radiation with a frequency of 8.6
Q38: How much energy is required to ionize