Multiple Choice
An orbital's orientation in space is determined by ________
A) the 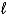 quantum number.
quantum number.
B) the ml quantum number.
C) the ms quantum number.
D) the n quantum number.
E) both the 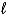 and ml quantum numbers.
and ml quantum numbers.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q88: Arrange the following elements in order of
Q89: The fact that the absorption and emission
Q90: Subshells are _<br>A)orbitals with the same principal
Q91: Which one of the following diagrams represents
Q92: A shell consists of all _<br>A)electrons with
Q94: Which atom or ion has the largest
Q95: Which prediction regarding the formation of a
Q96: Which listing has the orbitals in order
Q97: What is meant when two or more
Q98: Which statement about electromagnetic radiation is not