Multiple Choice
A shell consists of all ________
A) electrons with the same 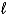 quantum number.
quantum number.
B) orbitals with the same quantum numbers.
C) orbitals with the same principal quantum number.
D) electrons with the same magnetic quantum number.
E) orbitals with the same 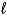 and ml quantum numbers.
and ml quantum numbers.
Correct Answer:

Verified
Correct Answer:
Verified
Q87: Place these types of radiation in order
Q88: Arrange the following elements in order of
Q89: The fact that the absorption and emission
Q90: Subshells are _<br>A)orbitals with the same principal
Q91: Which one of the following diagrams represents
Q93: An orbital's orientation in space is determined
Q94: Which atom or ion has the largest
Q95: Which prediction regarding the formation of a
Q96: Which listing has the orbitals in order
Q97: What is meant when two or more