Multiple Choice
Which listing has the orbitals in order of increasing energy in a multielectron atom?
A) 3s 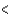 3p
3p 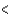 4s
4s 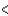 3d
3d 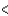 4p
4p
B) 3s 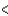 3p
3p 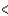 3d
3d 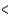 4s
4s 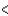 4p
4p
C) 3s 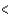 4s
4s 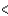 3p
3p 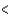 4p
4p 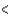 3d
3d
D) 3s 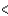 3px
3px 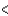 3py
3py 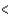 3pz
3pz
E) 2s 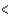 3s 2p
3s 2p 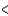 3d
3d
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q91: Which one of the following diagrams represents
Q92: A shell consists of all _<br>A)electrons with
Q93: An orbital's orientation in space is determined
Q94: Which atom or ion has the largest
Q95: Which prediction regarding the formation of a
Q97: What is meant when two or more
Q98: Which statement about electromagnetic radiation is not
Q99: Copper forms a cation with a charge
Q100: What is the ground-state electron configuration of
Q101: Which arrangement is correct for increasing atomic