Multiple Choice
Which of the following is not a possible set of quantum numbers for an electron?
A) n  3,
3, 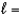 2, me
2, me
 1
1
B) n  2,
2, 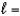 2, me
2, me  0
0
C) n  4,
4, 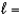 1, me
1, me  1
1
D) n  1,
1, 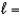 0, me
0, me  0
0
E) n  2,
2, 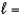 1, me
1, me  1
1
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q23: Indicate which of the following sources produces
Q24: Which of the following photons has the
Q25: Arrange the following elements in order of
Q26: Determine the wavelength of the line in
Q27: Give the names of the three quantum
Q29: Atomic spectra are due to the changes
Q30: Which arrangement is in the correct order
Q31: Which sequence does not correctly arrange the
Q32: Compared with the atomic radius of oxygen
Q33: An orbital's shape is determined by _<br>A)the