Multiple Choice
Compared with the atomic radius of oxygen (Z  8) , the atomic radius of sulfur (Z
8) , the atomic radius of sulfur (Z  16) is ________
16) is ________
A) larger because the n quantum number increases.
B) smaller because the atomic number (nuclear charge) increases.
C) smaller because the n quantum number increases.
D) larger because the 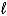 quantum number increases.
quantum number increases.
E) about the same because in both cases, the nuclear charge equals the number of electrons.
Correct Answer:

Verified
Correct Answer:
Verified
Q27: Give the names of the three quantum
Q28: Which of the following is not a
Q29: Atomic spectra are due to the changes
Q30: Which arrangement is in the correct order
Q31: Which sequence does not correctly arrange the
Q33: An orbital's shape is determined by _<br>A)the
Q34: Which subshell contains six total electrons?<br>A) <img
Q35: The change in energy of a one-electron
Q36: Which transition in a hydrogen atom will
Q37: Electromagnetic radiation with a frequency of 8.6