Multiple Choice
You heat a cup of coffee in a microwave oven. It absorbs 40 kJ of energy from the microwave, and the volume expands. Which one of the following statements correctly describes the relationship between the change in enthalpy H and the change in internal energy E of the coffee? ( means greater than, means less than)
A) (H  40 kJ, E
40 kJ, E 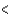 40 kJ)
40 kJ)
B) (H  40 kJ, E
40 kJ, E 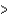 40 kJ)
40 kJ)
C) (H 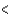 40 kJ, E
40 kJ, E  40 kJ)
40 kJ)
D) (H 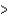 40 kJ, E
40 kJ, E  40 kJ)
40 kJ)
E) (H  40 kJ, E
40 kJ, E  40 kJ)
40 kJ)
Correct Answer:

Verified
Correct Answer:
Verified
Q58: Given the following thermochemical equation detailing the
Q59: How much work does a gas do
Q60: The kinetic energy associated with the random
Q61: Which of the following is an endothermic
Q62: C<sub>8</sub>H<sub>18</sub> (114 g/mol, d <font face="symbol"></font> 0.69
Q64: The best definition of the enthalpy change
Q65: If 7.3 kJ of energy are required
Q66: The capacity to do work is a
Q67: A pot of water is heated with
Q68: Determine the enthalpy of formation for glucose