Multiple Choice
If 7.3 kJ of energy are required to change the temperature of water from 5.0 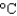 to 70.0
to 70.0 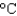 , what was the volume of water? (cs 4.184 J/(g
, what was the volume of water? (cs 4.184 J/(g 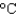 ) , d 1.00 g/mL)
) , d 1.00 g/mL)
A) 27 mL
B) 37 mL
C) 110 mL
D) 0.73 mL
E) 75 mL
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q60: The kinetic energy associated with the random
Q61: Which of the following is an endothermic
Q62: C<sub>8</sub>H<sub>18</sub> (114 g/mol, d <font face="symbol"></font> 0.69
Q63: You heat a cup of coffee in
Q64: The best definition of the enthalpy change
Q66: The capacity to do work is a
Q67: A pot of water is heated with
Q68: Determine the enthalpy of formation for glucose
Q69: Use the following information to determine the
Q70: In an experiment, 7.5 g of liquid